Abstract
BACKGROUND
Recently, utilization of triplet regimens featuring 2 novel agents, such as lenalidomide, bortezomib, and dexamethasone (RVd), has increased in multiple myeloma (MM). RVd as well as cyclophosphamide, bortezomib, and dexamethasone (CyBord) are the most common triplet regimens used in first-line (1L), newly diagnosed MM (NDMM) patients (pts). Using a large electronic health records (EHR) database, this study aimed to assess real-world outcomes among pts with NDMM who do not receive a stem cell transplant (SCT) initiating RVd or CyBord.
METHODS
A retrospective observational study of NDMM pts was conducted utilizing EHR from a nationally representative database (Flatiron Health). The Flatiron MM provider network comprises over 265 clinics throughout the United States. Pts with an ICD-9 (203.0x) or ICD-10 (C90.xx) diagnosis of MM between 01/01/2011 and 05/31/2017 were randomly selected into the study. Diagnosis of MM was confirmed through review of unstructured chart data (eg, physician notes). Index date was defined as the pt's date of MM diagnosis. Pts were excluded if they did not have ≥ 2 documented clinical visits during the study period, received MM treatment (Tx) > 14 days prior to their first diagnosis date, or had evidence of SCT.
Start of 1L therapy was defined as the first episode of an eligible systemic Tx given after or up to 14 days before the index date. Regimens were defined using the first eligible drug episode plus other eligible drugs given within 28 days of each other. A maximum gap of 180 days was allowed within a given line of therapy (LOT). Duration of therapy (DOT) was defined as the time from start of 1L until discontinuation of the last agent of the 1L regimen. Time to next treatment (TTNT) was defined as the duration from the start of 1L until the initiation of another LOT. Treatment-free interval (TFI) for pts who progressed to 2L was defined as TTNT minus DOT. Kaplan-Meier method was used to calculate median TTNT, and Cox proportional hazards model was developed to evaluate risk factors for TTNT.
RESULTS
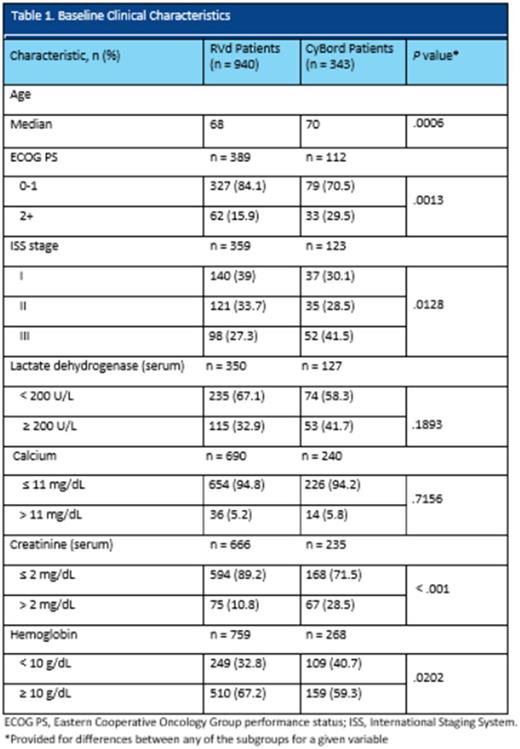
Of the 6,245 MM pts in the database, 940 received RVd and 343 received CyBord in the 1L. There were no significant differences in gender or race between the groups, but RVd pts were younger (68 vs 70, P= .0006) and were more likely to be treated in a community practice (93.8% vs 88.3%, P= .001). RVd pts had lower ECOG scores (P= .0098) and ISS staging (P= .0128), although approximately 60% of pts had missing data for each variable. CyBord pts had more kidney involvement (serum creatinine > 2 mg/dL in 28.5% vs 10.8%, P < .001) and anemia (Hgb < 10 g/dL in 40.7% vs 32.8%, P= .0202) (Table 1).
Fewer RVd pts progressed to a second LOT during the study period (n = 299, 24.4% vs n = 129, 37.6%, P < .0001). Both median DOT and median TTNT were longer among those treated with RVd 10.6 and 38.6 mos (95% CI: 32.5, 44.6) vs CyBord 8.6 (P= .14) and 20.4 mos (95% CI: 15.0, 24.4, P < .0001) respectively. Among pts who progressed, the TFI for RVd vs CyBord was 29 vs 34 days (P= .08).
After controlling for pt and disease characteristics at baseline, factors associated with higher probability of advancing to 2L were receipt of CyBord (ref: RVd, HR: 1.65, 95%: 1.32, 2.06, P < .0001), presence of sleep disorders (HR: 2.19, 95%: 1.02, 4.69, P= .0448), and presence of IgM (HR: 5.64, 95%: 2.32, 13.73, P= .0001). Notable predictors of lower risk of advancing to the 2L were treatment at a community practice (HR: 0.67, 95%: 0.48, 0.94, P= .0216) and serum albumin ≥ 3.5 g/dL (ref: < 3.5 g/dL, HR: 0.75, 95%: 0.58, 0.99, P= .0416).
CONCLUSION
The SWOG S0777 study reported a cumulative DOT of 18.7 mos (RVd induction for 6 mos and Rd maintenance for 12.7 mos) and a median PFS of 43 mos. In this study, patients with NDMM treated with RVd in a real-world setting had a median DOT of 10.6 mos and TTNT of 38.6 mos, which was 56.7% and 89.7% of those reported in the SWOG S0777 study, respectively. RVd was used more frequently than CyBord and after controlling for baseline characteristics, the risk of progressing to the 2L of therapy remained higher for patients on CyBord. The observed shorter DOT and PFS as well as the relationship between community practice and lower risk of progressing to the next line may be due to more regimented treatment-to-progression approaches in clinical trials and a reflection of the different treatment approaches at academic vs community centers with regard to the frequency of monitoring and addition or switch of MM therapy.
Chari: Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Research Funding; Bristol-Myers Squibb: Consultancy, Other: Research funding (to AC's institution); travel, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Research Funding; Millennium: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Research Funding; Acetylon Pharmaceuticals: Other: Research funding (to AC's institution); Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Research Funding; Array BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Research Funding; Onyx: Research Funding; Biotest: Other: Research funding (to AC's institution), Research Funding; Pharmacyclics: Research Funding. Ung: Celgene Corporation: Other: Fellowship is funded by Celgene. Abouzaid: Celgene Corporation: Employment, Equity Ownership, Research Funding. Ni: Celgene: Employment. Parikh: Celgene Corporation: Employment. Agarwal: Celgene Corporation: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal